Why Is Outer Space Cold?
Author; ROGELIO PEREZ CASADIEGO
Why is the outer space cold? If it is assumed to be mostly empty and temperature is a characteristic of the energy level of matter. All vacuum due to its lack of substance is a thermal insulator, but it is well known that in outer space astronauts when protected by the shade of our planet may experience temperatures of -180°C, but when they are facing the radiation of the sun the temperature they can withstand is 120°C. Of course this is possible thanks to space suits, now for thermal conduction a substance is necessary, by which the outer space should not be cold if this vacuum, Then we wonder what substance fills the outer space by originating its cold? Since the entire space of the universe is very cold, which has approximate temperatures from -180°C to almost 0 k. to answer this question we must know that the universe in its beginning was quite the opposite, this was very hot, due to the ion soup of the great plasma that filled everything, but when these particles began to cool down, originated the dark age of the universe due to an ion gas that filled everything, but then a mystery happened in the universe, the particles of the gas that filled the universe became transparent, knowing that matter is not created or destroyed, but only transformed, we can affirm that the same ions that originated the high temperatures due to the plasma state in which they appeared, now by phase changes, they are transformed into an ionic solid state, transparent matter that originates the cold of outer space. So we can say that the universe is full of frozen ions, or ionic crystals.
Keywords; plasma, ion gas, ionic crystals, ions, phase changes.
Introduction:
According to the big bang theory, space and matter appeared together (universe), but matter within it was a hot plasma and as the universe expanded, matter cooled down, we all know that any substance or mixture by modifying its temperature or pressure conditions, gets different states or phases, we know according to the scientific explanations, that these conditions occurred in the primitive universe, which led to a change of phase of matter, going from a very hot ionized gas, to a much colder gas, when these conditions began to occur, this gas originated the dark age of the universe, but over time a mystery happened, matter became transparent, and the universe moved from dark to transparent age, as matter is not created, nor destroyed, only transformed, so we can safely say, that matter did not disappear, but change to the transparent phase and the universe came to make a very cold place, now the transparent phase of an ionic gas, is the solid phase.
Definitions;
In physics and chemistry, it is observed that for any material substance or element, changing its temperature or pressure conditions, different states or phases, called states of matter aggregation, can be obtained in relation to the binding forces of the particles (molecules, atoms or ions) that constitute it.
The matter is presented to us in various states of aggregation, all with different properties and characteristics, and although the most known and observable daily are four, the so-called solid, liquid, gaseous and plasma phases, there are also other states observable under extreme conditions of pressure and temperatura.1
Cold; from Latin frig-dus, or rather, the lack of heat is defined according to the RAE as that body that has a much lower temperature than that of the ordinary environment.2
What is temperature? It is a magnitude that refers to the common notions of heat, cold, warm or warm, measurable with a thermometer. In physics, it is defined as a magnitude scale related to the internal energy of a thermodynamic system, defined by the zero of thermodynamics. More specifically, it is directly related to the part of the internal energy known as "kinetic energy", which is the energy associated with the movements of the particles in the system, is in a sense translational, rotational or in the form of vibrations. . Temperature is usually measured in degrees Celsius (°C), and also in degrees Fahrenheit (°F) or with an absolute temperature unit such as Kelvin (K). The absolute zero (0 K) corresponds to where the absolute zero is at least 273.15 degrees Celsius.3
An astronaut orbiting around Earth, the temperature can vary abruptly in a matter of seconds, depending on what is facing the Sun or protected by the shadow of our planet. In the latter case, the temperature can reach -180o C. However, if the astronaut is going to face the king of the sun, the heat becomes unbearable, reaching 122 ºC, but as we move away from the sun this temperature decreases, so theoretically the lowest temperature in space would be 0 ºK (-273.15 ° C), when there is no energy.4
Absolute zero is the lowest possible theoretical temperature. At this temperature the energy level of the internal system is the lowest possible, so the particles, according to classical mechanics, lack movement; 5 However, according to quantum mechanics, absolute zero must have a residual energy, called zero point. Energy, and thus be able to comply with the Heisenberg Uncertainty Principle, absolute zero serves as a starting point for both the Kelvin scale and the Rankine scale.6
What are crystals? It comes from the Greek word krystallos. Initially came the name "kryos" which means cold, alluding to the formation of ice from water. Later the name changed in connotation referring rather to transparency, so the Greeks gave the name "krystallos" of quartz, initially believing that it was a variety of ice that was not liquefied at temperature ambiente.8
The entropy of a pure and perfect ideal crystal would be zero. If the atoms that make up it do not form a perfect crystal, its entropy must be greater than zero, so the temperature is always greater than absolute zero and the crystal always has imperfections induced by the movement of its atoms, a movement that the compensated and, therefore, always having a residual imperfection.
One of the best thermal insulators is a vacuum, in which heat is only emitted by radiation, but due to the great difficulty in obtaining and maintaining vacuum conditions is rarely used.9
Results;
The outer space is a very cold place, and besides in this are all the things that exist, some may consider that the outer space is mostly empty, but a vacuum is a thermal insulation, because it has no matter, but the outer space is a very cold place, so why is it cold? the answer is because it is full of the same matter with which it emerged in the big bang, and this matter are ions, (outer space was born full of ions in plasma state), we also know that any substance, modifying its temperature or pressure conditions, changes phase or aggregate state, and that is the cause that ion matter, that filled the universe is not a hot soup, but is now in solid ionic form.
What process happened in the evolution of the ions in the plasma state to the solid state?
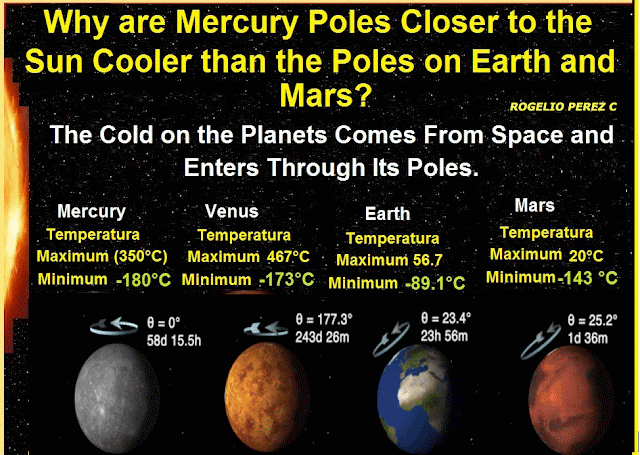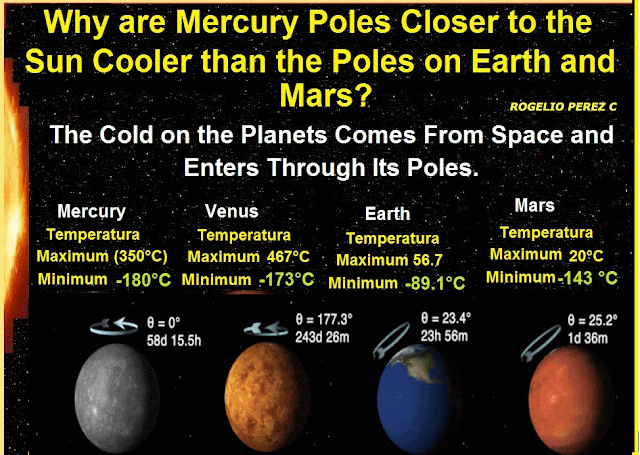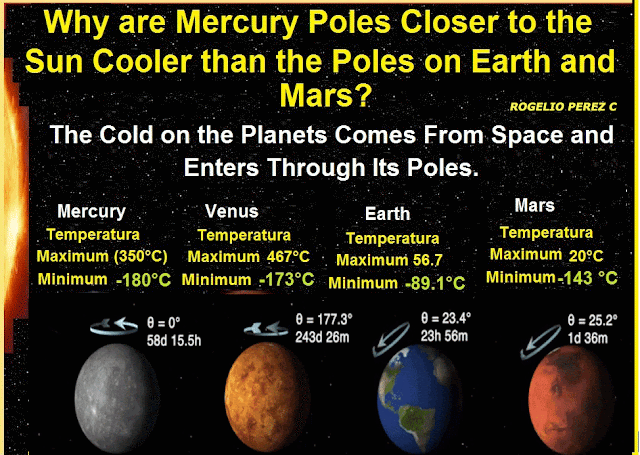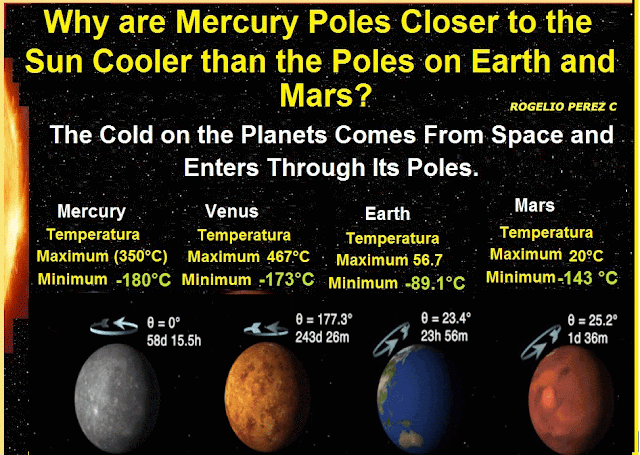
The first process was Deionization: It is the change of plasma to a cooler gas; the second process was Reverse Sublimation, i.e. the direct passage from the gaseous state to the solid state, but when an ionic gas evolves toward a solid. In this phase the ionic matter is crystalline, and the crystals are the only matter with entropy to reach the lowest temperatures we observe deep in the outer space. Now if the outer space is filled with ionic crystals that originate the cold, eventually this matter will try to leak into the planets in it, and according to this research, these ionic crystals are filtered to the planets through their poles, because they are the coldest places on the planets.
Conclusion; it can be said that the cold in outer space is produced by solid-state ions (ion crystals), in other words frozen ions fill the universe causing its cold, these appeared next to the space time in the state of a hot soup (plasma that fills the universe), in addition, substances or matter at temperatures that we observe in outer space tend to crystallize, so too crystals are the only material that by their characteristic can produce the low temperatures that we observe in outer space. Finally it is concluded that the cold on the planets comes from outer space, and enters these through its poles.
Bibliography
1. https://ceramica.fandom.com/wiki/Estado_de_agregaci%C3%B3n_de_la_materia
2. https://dle.rae.es/fr%2525C3%2525ADo
3. https://es.wikipedia.org/wiki/Temperatura
4. https://www.muyinteresante.es/curiosidades/preguntas-respuestas/ique-temperatura-hay-en-el-espacio-exterior
5. Pierre J. Rapin – Prontuario del frío, p. 5, en Google Libros
6. von Reichenbach, María Cecilia; Bergero, Paula Elena; Álvarez, Ariel; del Río, Laura. Reichenbach, María Cecilia von, ed. Cero absoluto. Curiosidades de física. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). p. 104. ISBN 978-950-692-088-3.
7. Jerry D. Wilson, Anthony J Buffa – Física, p. 354, en Google Libros
8. https://es.wikipedia.org/wiki/Cristal#cite_note-Online_Dictionary_of_Crystallography-1
9. https://es.wikipedia.org/wiki/Aislante_t%C3%A9rmico

Well that's the dumbest thing I've heard all day.
ResponderEliminarWhat drives you to think that a tenuous neutral (or partially ionized) gas is in any way crystalline?